© A.W.Marczewski 2002
A Practical Guide to Isotherms of ADSORPTION on Heterogeneous Surfaces
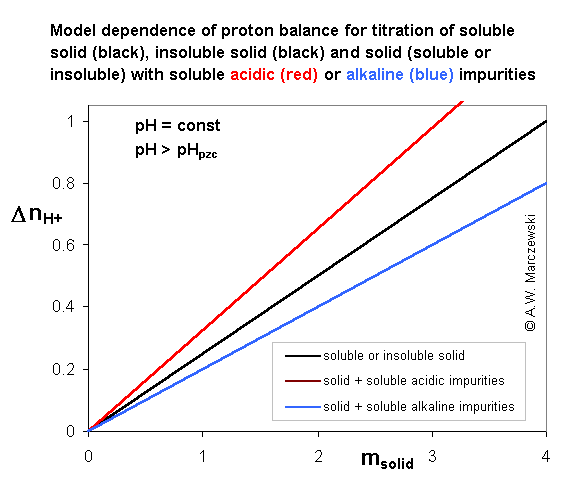
System I (assumed complete solubility or insolubility)
Insoluble solid (e.g. like titania) or soluble solid (like citric acid) containing 2 types of soluble (or insoluble) affecting pH impurities.
Co-ordinates: proton balance (excess/deficit of protons caused by the presence of solid in given electrolyte at given fixed pH value - in this model case: pH > pHpzc), ΔnH+ vs. mass of titrated solid.
Surface charge may be determined from the slope of line: proton balance, ΔnH+ vs. mass of titrated solid (see equations)
Soluble or insoluble solid - no saturation; for a fixed pH > pHpzc the proton balance is directly proportional to sample mass. Small amounts of chemically neutral impurities (e.g. NaCl) should not affect proton balance, but only if specific ion adsorption may be neglected and ionic strength does not change significantly (the change of ionic strength affects surface charging).
Soluble (or insoluble) acidic impurity - no saturation; for a fixed pH > pHpzc the proton balance is directly proportional to sample mass (slope is higher than for pure solid).
Soluble (or insoluble) alkaline impurity - no saturation; for a fixed pH > pHpzc the proton balance is directly proportional to sample mass (slope is lower than for pure solid).
System II (assumed partial solubilities)
Partially soluble solid (e.g. like silica or alumina for high pH) containing 2 types of partially soluble (a affecting pH) impurities.
Co-ordinates: proton balance (excess/deficit of protons caused by the presence of solid in given electrolyte at given fixed pH value - in this model case: pH > pHpzc), ΔnH+ vs. mass of titrated solid.
Surface charge may be determined from the slope of line: proton balance, ΔnH+ vs. mass of titrated solid (see equations) for large masses - above saturation points.
Impurity 1 (or products of its reaction with substances present in solution) reaches saturation as the 1st.
Impurity 2 (or products of its reaction with substances present in solution) reaches saturation as the 2nd.
Solid (or products of its reaction with substances present in solution) reaches saturation as the last.
Surface charge may be determined from the slope of line: proton balance, ΔnH+ vs. mass of titrated solid (see equations)
Send a message to Adam.Marczewski AT@AT umcs.lublin.pl