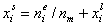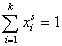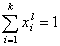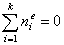© A.W.Marczewski 2002
A Practical Guide to Isotherms of ADSORPTION on Heterogeneous Surfaces
Reload Adsorption Guide
Adsorption basics:
Liquid mixtures
Measured quantity:
Adsorbed amount vs. Adsorption excess
Basics of liquid adsorption: binary liquid adsorption
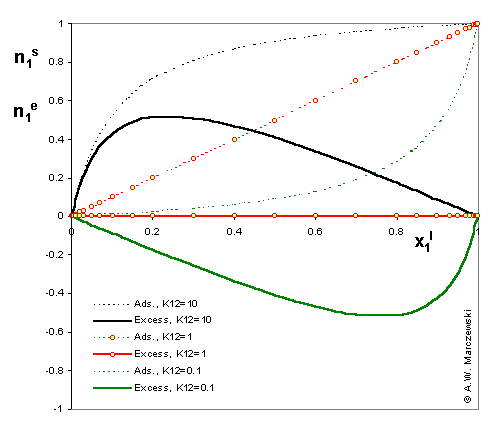
The picture shows comparison of surface excess, n1e (solid lines -----) and respective adsorption n1s values (dotted lines .....) for model Everett isotherm (homogeneous surface, ideal surface and bulk phase) and 3 values of adsorption constant K12 = K1 / K2 : 10 (stronger adsorption of "1" - positive excess of "1"), 1 (equal adsorption properties of "1" and "2" - adsorption excess is 0 everywhere) and 0.1 (stronger adsorption of "2" - negative excess of "1" and positive excess of "2")
See Note on molecular sizes
Measured adsorption quantity: always
surface excess, nie related to true adsorption nis (or ai) by following dependences (symmetrical with respect to component exchange):
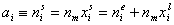 or
or
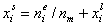
where superscript "l" denotes liquid, "s" surface phase, xi is molar fraction of component "i", ns is adsorption (nis) and ne is surface excess (nie) being the total excess of component "i" in surface phase vs. bulk phase per amount of adsorbent (or adsorbent surface).
For any multicomponent system we have by definition:
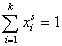
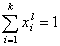
and (for equal molecular sizes - see Note):
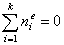
Adsorption basics:
Gas ( Basics )
Non-electrolyte liquids ( Basics, Everett iso., linear plots, heterogeneity )
Dilute solutions ( Dilute solutions )
Top
E-mail addresses are modified to in order to prevent spamming / mail-abuse:
in e-mail remove spaces, replace " AT@AT " by "@"
Send a message to Adam.Marczewski AT@AT umcs.lublin.pl
Disclaimer
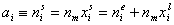 or
or