© A.W.Marczewski 2002
A Practical Guide to Isotherms of ADSORPTION on Heterogeneous Surfaces
Reload Adsorption Guide
ADSORPTION:
Prediction of multicomponent adsorption and
Heterogeneity and molecular size
(some ideas)
General Integral Equation /
GL (Generalized Langmuir) /
All equations (preview)
Adsorption type (
Linear Langmuir plot /
Graham plot /
Consistency /
Henry constant )
Popular isotherms
(
Mono-,
Multilayer,
Experimental,
Micro-,
Mesoporous
)
Data analysis:
LSq data fitting /
Heterogeneity: Global ,
σE /
Linear plots /
φ-function /
Pores
)
Prediction/Description of
Multicomponent adsorption /
Wastewater adsorption
Heterogeneity and Molecular Size ( Theory and Prediction / Simple binary isotherm )
Data analysis - prediction of multi-component adsorption:
-
 Prediction of multicomponent adsorption for components with non-linearly correlated adsorption energy distributions.
(Here are model pictures)
Prediction of multicomponent adsorption for components with non-linearly correlated adsorption energy distributions.
(Here are model pictures)
Proposed method assumes that a certain correlation (generally non-linear) exists between adsorption energies of various adsorbates on a solid surface. Generally no limit of no. of components exists. (Necessary theoretical background is given in multi-component GIEA and Energy correlation sections). Prediction requires data for simple (single-component or binary) systems or at least estimated values of heterogeneity parameters or energy dispersion σE values.
For more info consult these papers:
- "Unified Theoretical Description of Physical Adsorption from Gaseous and Liquid Phases on Heterogeneous Solid Surfaces and Its Application for Predicting Multicomponent Adsorption Equilibria", A.W.Marczewski, A.Derylo-Marczewska and M.Jaroniec, Chemica Scripta, 28, 173-184 (1988) (pdf, hi-res pdf available upon e-mail request).
- "Correlations of Heterogeneity Parameters for Single-Solute and Multi-Solute Adsorption from Dilute Solutions", A.W.Marczewski, A.Derylo-Marczewska and M.Jaroniec, J.Chem.Soc.Faraday Trans.I, 84, 2951-2957 (1988),
(doi).
- "A Simplified Integral Equation for Adsorption of Gas Mixtures on Heterogeneous Surfaces", A.W.Marczewski, A.Derylo-Marczewska and M.Jaroniec, Mh.Chem., 120, 225-230 (1989),
(doi).
- "Prediction of the Heterogeneity Parameters for Adsorption of Multicomponent Liquid Mixtures on Solids", A.W.Marczewski, A.Derylo-Marczewska, M.Jaroniec and J.Oscik, Z.phys.Chem., 270(4), 834-838 (1989) (pdf, hi-res pdf available upon e-mail request).
- "Analysis of Experimental Data for Adsorption of Organic Substances from Dilute Aqueous Solutions on Activated Carbon", A.Derylo-Marczewska and A.W.Marczewski, Polish J. Chem., 71, 618-629 (1997)
- "A General Model for Adsorption of Organic Solutes from Dilute Aqueous Solutions on Heterogeneous Solids: Application for Prediction of Multisolute Adsorption", A.Derylo-Marczewska and A.W.Marczewski, Langmuir, 13, 1245-1250 (1997),
(doi).
This method may predict adsorption (or at least is able to estimate the magnitude of heterogeneity effects) in many situations:
- Prediction of adsorption in gas mixtures (required: single gas adsorption data for all components; useful: binary gas mixture data - if e.g. prediction of tertiary-mixture adsorption is wanted; quality of prediction - very good).
- Prediction of adsorption in multi-component dilute solutions (required: single solute adsorption data for all components; useful: binary dilute solute adsorption data - if e.g. prediction of tertiary mixture adsorption is wanted; quality of prediction - good or very good).
- Prediction of adsorption in liquid mixtures (required: single gas/vapour adsorption data for all components; useful: binary gas mixture data - if e.g. prediction of tertiary-mixture adsorption is wanted; quality: heterogeneity parameters are nicely estimated, other parameters require correction) (for the prediction in e.g. tertiary systems, it is much better if binary liquid mixtures are available).
See prediction steps
-
 Prediction of adsorption in wastewater - unlimited no. of components and variable composition.
(Here are model pictures)
Prediction of adsorption in wastewater - unlimited no. of components and variable composition.
(Here are model pictures)
Proposed method assumes that all adsorbates are similar in chemical properties and their distributions af adsorption energies have the same shape but different position on energy axis (same heterogeneities, different adsorption equilibrium constants). Generally no limit of no. of components exists. Prediction requires data for simple (single-component or binary) systems.
In the light of the above presented prediction method as well as a heterogeneity vs. molecular size and heterogeneity study it is crude oversimplification. However, the usefulness of this approach may overcome its limits.
E.g. for wastewater treatment, where usually a large and only partially known no. and kind of pollutants is present. In such a case, if only an approximate mixture-profile is known, the adsorption of the entire mixture may be quite well predicted. In this way the right amount of adsorbent (e.g. active carbon) may be determined with a narrow margin error.
For more info consult these papers:
- "A Method of Investigation of Adsorption Equilibria on Active Carbons",
A.W.Marczewski, A.Derylo-Marczewska and M.Jaroniec, Ochrona Srodowiska, 521/2-3(32-33), pp. 37-42, PZITS, Wroclaw (1987) (paper and oral presentation in Polish),
-
"A Simple Method for Describing Multi-Solute Adsorption Equilibria on Activated Carbons", A.W.Marczewski, A.Derylo-Marczewska and M.Jaroniec, Chem.Engng.Sci., 45(1), 143-149 (1990),
(doi).
 Heterogeneity vs. molecular size and topography:
Heterogeneity vs. molecular size and topography:
-
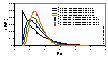
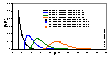 Theoretical approach based on simple statistics.
(Here are model pictures)
Theoretical approach based on simple statistics.
(Here are model pictures)
The proposed approach does not assume any particular adsorption isotherm, though localised physical adsorption in monolayer is implied. It is assumed, that the observed heterogeneity comes from the entire system:
- adsorbate with its specific properties (in the simplest case it is a polymer-like compound built of "mers" having the same size as surface sites)
- adsorption sites with their topography (neighbour-neighbour correlations - conditional probabilities) (e.g. pure random, patchwise, mixed, chessboard-like etc.)
- "effective" surface topography may also include some adsorbate-factor as not all possible combinations of adsorbate-mers - surface-sites are possible depending on the compound flexibility (polymer-like or stick-like etc.)
- as a heterogeneity measure, the adsorption energy dispersion is used - it is calculated by using all posssible energies of adsorption with their respective probabilities; in fact it is possible that in many situations (e.g. large molecules) not all possiblities may be observed due to site-screening effects (the weakest site-combinations may never be occupied), but here heterogeneity shows only a potential for differences in energies.
For more info consult this paper:
- "Energetic Heterogeneity and Molecular Size Effects in Physical Adsorption on Solid Surfaces", A.W.Marczewski, A.Derylo-Marczewska and M.Jaroniec, J.Colloid Interface Sci., 109, 310-324 (1986),
(doi).
(This paper is probably my most-often cited one and I must say it gave me a lot of fun to find all this!)
This approach explained several observed facts and helped to reject some wide-spread (at that time) myths.
- Adsorption energy is proportional to the "size" (observed e.g. for experimental adsorption data of n-alkanes) - always true! - does not depend on topography. It is true for:
- minimum adsorption energy
- average adsorption energy
- maximum adsorption energy
- Myth: random topography means larger heterogeneity - false! - for larger molecules surfaces with the same structural (i.e. "site") heterogeneity with patchwise topography show higher heterogeneity effects than those with random topography
- for patchwise topography the dispersion of adsorption energy is proportional to the molecular size
- for random topography the dispersion of adsorption energy is proportional to the square root of molecular size (energy variancy is proportional to the molecular size)
- for regular/ordered topographies (e.g. checkerboard topography) the observed heterogeneity will change periodically with molecular size
-
Simple equation of adsorption on heterogeneous solids from binary mixtures of gases or bi-component dilute solutions for components with different molecular sizes (for gas adsorption: M.Jaroniec, Thin Solid Films, 81 L97 (1981)).
Read more in:
- "Competitive Adsorption of Binary Gas Mixtures on Energetically Heterogeneous Solids", M.Jaroniec and A.W.Marczewski, Thin Solid Films, 92, 385-391 (1982),
(doi).
- "An Equation for Multi-Solute Adsorption from Dilute Aqueous Solutions Involving Energetic Heterogeneity of the Solid and Differences in Molecular Sizes of the Solutes", M.Jaroniec, A.Derylo and A.W.Marczewski, Chem.Engng.Sci., 38, 307-311 (1983),
(doi).
For adsorption system characterised by quasi-gaussian energy distribution and heterogeneity coefficient m and components with size ratio r12 = r1 / r2 in conditions where adsorbed layer is almost filled-up :
K12 =
[a1 / a2 r12 ]1/m [c2 r12 / c1]
or in forms useful for data presentation:
[a1 / a2 r12 ] = { K12 [c1 / c2 r12] } m
[a1 / c1m ] = K12m [a2 / c2m]r12
In logarythmic form this equation is easier to use in data analysis and presentation:
-
ln(a1) - r12 ln(a2) = m ln(K12) + m [ln(c1) - r12 ln(c2)]
-
ln(a1) - m ln(c1) = m ln(K12) + r12 [ln(a2) - m ln(c2)]
The most useful forms are:
-
ln(a1 / a2r12) = m ln(K12) +
m ln(c1 / c2r12)
-
ln(a1 / c1m) = m ln(K12) + r12 ln(a2 / c2m)
This approach worked well for many gas mixtures and binary dilute solutions even for low concentrations/adsorptions, where the condition of almost complete monolayer filling cannot be met.
Certain weakness of this equation is fitting procedure - though there are apparently only 2 straight line parameters (m ln(K12) and m or m ln(K12) and r12) one of the remaing parameters must always be included in fitted variables (r12 or m, respectively) in fitted line x,y-data. So in fact raw adsorption data (a1, a2, c1, c2) should be optimised, with appropriate statistical weights. In such a case, the above linear forms may serve as a valuable illustration/presentation tool.
NOTE.
The meaning of heterogeneity parameter m (for 2 components - or even 3 for liquid adsorption - with different energy distributions - is it m1 m2 or may be m12 - relative heterogeneity of "1" and "2" - or sth. else?) or underlying surface topography may be explained within Theoretical Approach above.
Adsorption type (
Linear Langmuir plot /
Graham plot /
Consistency /
Henry constant )
Popular isotherms
(
Mono-,
Multilayer,
Experimental,
Micro-,
Mesoporous
)
Data analysis:
LSq data fitting /
Heterogeneity: Global ,
σE /
Linear plots /
φ-function /
Pores
)
Prediction/Description of
Multicomponent adsorption /
Wastewater adsorption
Heterogeneity and Molecular Size ( Theory and Prediction / Simple binary isotherm )
General Integral Equation /
GL (Generalized Langmuir) /
All equations (preview)
Top
My papers
Search for papers
Main page
E-mail addresses are modified to in order to prevent spamming / mail-abuse:
in e-mail remove spaces, replace " AT@AT " by "@"
Send a message to Adam.Marczewski AT@AT umcs.lublin.pl
Disclaimer
 Prediction of multicomponent adsorption for components with non-linearly correlated adsorption energy distributions.
(Here are model pictures)
Prediction of multicomponent adsorption for components with non-linearly correlated adsorption energy distributions.
(Here are model pictures)
 Prediction of adsorption in wastewater - unlimited no. of components and variable composition.
(Here are model pictures)
Prediction of adsorption in wastewater - unlimited no. of components and variable composition.
(Here are model pictures)


