Physical consistency conditions require:
- minimum and maximum adsorption energies (not fulfilled for most analytical isotherms of adsorption on heterogeneous surfaces - see below)
- isotherm with Henry region (results from max. energy condition) - if an isotherm conforms to this requirement (e.g. Tóth, Gauss) it does not mean that it fulfills the 1st one (min/max energies) - see below
Henry constant KH may be defined as:
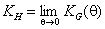 where θ = a/am
where θ = a/am or alternatively:
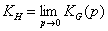 or
or
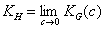
where KG(θ) is Graham's equilibrium function:
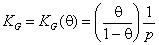 for gas adsorption
for gas adsorption
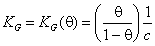 for dilute solute adsorption
for dilute solute adsorption
This condition is in fact equivalent to:
limp→0(φ) = 1
or
limc→0(φ) = 1
where the so-called φ-function is:
φ = δ log(a)/
δ log(p)
or
φ = δ log(a)/
δ log(c)
1/θt,H(p) = 1/(KHp) + 1/θt(p) (gas phase)
or
1/θt,H(c) = 1/(KHc) + 1/θt(c) (solute adsorption)
The resulting isotherm will display the Henry behavior.
CAUTION! This method cannot be used to force Henry behavior for DR or DA isotherms.
If the original isotherm equation θt(p) ( or θt(c) ) displays monolayer behavior, the resulting isotherm will have such plateau, too.
The Langmuir behavior (see below) may also be forced by an analogous "trickery".
In a similar manner a Langmuir-like behaviour (see below) at p → ∞ may also be obtained.
Langmuir constant KL may be defined as:
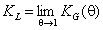 or
or
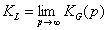 or
or
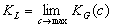
A detailed discussion and mathematical formulation for Henry and (above formulated) Langmuir constants may be found in the Appendix of the paper:
- "Unified Theoretical Description of Physical Adsorption from Gaseous and Liquid Phases on Heterogeneous Solid Surfaces and Its Application for Predicting Multicomponent Adsorption Equilibria", A.W.Marczewski, A.Derylo-Marczewska and M.Jaroniec, Chemica Scripta, 28, 173-184 (1988) (pdf, hi-res pdf available upon e-mail request).
Physical consistency condition
It is usually believed, that the very existence of such a limit (i.e. isotherm tends to Henry behavior at very low adsorptions) is a consequence of the existing maximum of adsorption energy and is sometimes called a physical consistency condition. Though the maximum energy value always produces Henry behavior, the opposite is not necassarily true. It is easy to prove that it is enough that the energy distribution function defined in the infinite energy range (-∞ , +∞) behaves in a special way and such a limit is obtained (e.g. for Toth or RP isotherm equations).